Learning Outcomes
Students will be able to:
i. Define heat flow and work as two fundamental modes of energy transfer between systems.
ii. Explain the concept of heat flow, emphasizing its dependence on a temperature difference and its direction from hotter to colder systems.
iii. Differentiate between heat flow and work, recognizing that work involves the application of force against a resistance.
iv. Apply the concept of heat flow to calculate the amount of heat transferred using the equation Q = mcΔT, where Q represents the heat transferred, m is the mass of the substance, c is its specific heat, and ΔT indicates the change in temperature.
Introduction
Our world is a symphony of energy, a continuous dance of different forms that power our existence. Among these forms, heat flow and work play pivotal roles in shaping our experiences and influencing various natural phenomena. In this lesson, we delve into the fascinating realm of energy transfer, exploring the mechanisms by which heat flow and work enable the exchange of energy between systems.
i. The Flow of Heat: A Symphony of Temperature Differences
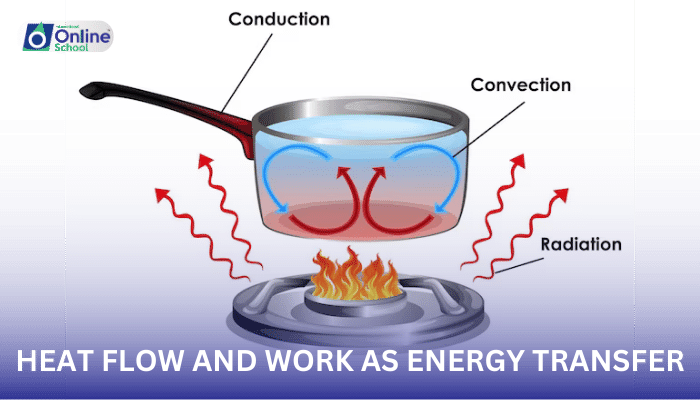
Imagine placing a hot spoon into a bowl of cold ice cream. The warmth of the spoon, a manifestation of its higher temperature, triggers a transfer of thermal energy, known as heat flow. As heat flows from the spoon to the ice cream, a gradual temperature exchange occurs, ultimately leading to a state where both objects approach the same temperature.
Heat flow occurs due to a temperature difference between two systems. This difference in temperature drives the random motion of molecules in the hotter system, causing them to collide with molecules in the colder system. These collisions transfer energy from the hotter molecules to the colder ones, resulting in a net flow of heat from the hotter system to the colder system.
ii. The Force of Work: Energy Transfer in Action
Work, another fundamental mode of energy transfer, involves the application of a force against a resistance. When we lift a book against the force of gravity, we perform work. The energy we expend in lifting the book is transferred to the book, increasing its potential energy. Similarly, when we push a car against the force of friction, we perform work, transferring energy to the car, causing it to move.
Work is distinct from heat flow in that it does not require a temperature difference. Energy transfer through work can occur even at constant temperature. For instance, compressing a gas involves performing work, increasing its internal energy, while expanding a gas results in work being done on the surroundings, decreasing its internal energy.
iii. Calculating Heat Flow: Quantifying Energy Transfer
The amount of heat transferred between systems can be calculated using the equation:
Q = mcΔT
where:
- Q represents the heat transferred (in joules)
- m is the mass of the substance (in kilograms)
- c is the specific heat of the substance (in joules per kilogram per Kelvin)
- ΔT indicates the change in temperature (in Kelvin)
Specific heat is a material property that determines the amount of heat required to raise the temperature of a unit mass of the substance by one Kelvin. Different substances have different specific heats, reflecting their unique abilities to store and transfer heat.
iv. Heat Flow and Work in Our Daily Lives: A Symphony of Energy Exchange
Heat flow and work play crucial roles in our daily lives, influencing various phenomena and technological advancements:
Cooking and Heating: Cooking relies on the transfer of thermal energy to transform food. Heat from a stovetop or oven conducts through the cookware, warming the food and causing chemical changes that lead to its preparation. Similarly, heating systems utilize the principles of heat flow to transfer thermal energy from a source to our homes or workplaces, maintaining comfortable indoor temperatures.
Mechanical Processes: Work is essential in various mechanical processes, including lifting objects, operating machines, and moving vehicles. The energy transferred through work enables us to perform tasks, manipulate objects, and accomplish a vast range of mechanical actions.
Biological Processes: Heat flow and work are crucial for various biological processes, such as maintaining body temperature, regulating enzyme activity, and enabling the growth of plants. Our bodies constantly strive to maintain thermal equilibrium with their surroundings, and work is performed by muscles to generate movement and perform actions.
Heat flow and work, fundamental modes of energy transfer, shape our world in countless ways. Their understanding is essential in fields as diverse as physics, engineering, biology, and everyday life. As we continue to explore the world around us, heat flow and work remain guiding principles, illuminating the path to new discoveries and technological advancements.